時間がない人向け
コピペで使えて、SMILESのテーブルデータから色々なデータを一発で生成するpythonクラスを書きました。
MOLオブジェクト、RDKit記述子、原子位置情報(deepchemで言うところのSmilesToImage)に対応しています。
他の記述子とかも同じ要領で書けるはずです。
個人的な備忘録なのでツッコミどころ多くても許してください。
はじめに
ケモインフォマティクスは化合物の情報とコンピュータを用いて様々な課題を解決しようとする学問領域です。
近年の機械学習などの手法の発展により、化合物情報を用いて物性を高度に予測できるようになってきました。
最近ではAlphaFold2の躍進などが有名1。
SMILESは有名・簡易な化合物表記方法の一種で、人間とコンピュータが共に理解しやすい文字列で化合物を表現します2。
今回はSMILESを用いたケモインフォマティクスコンペティションに参加したので、その備忘録として、役立ちそうなスクリプトをアウトプットすることにしました。
実装
- Python: 3.7.4
- rdkit: 2019.09.3.0
- numpy: 1.18.1
- pandas: 1.0.1
from dataclasses import dataclass, field
from typing import ClassVar, List, Dict, Tuple
import sys
import csv
from tqdm import tqdm
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
from rdkit import Chem
from rdkit.Chem import AllChem
from rdkit.Chem import Descriptors
from rdkit.Chem import MolStandardize
from rdkit.ML.Descriptors import MoleculeDescriptors
@dataclass
class data_loader:
# 実験用
data_file: str # ファイルパス
task: str # task名
preprocessing: bool = False # MOLオブジェクト前処理一括変更用
normalize: bool = True # MOLオブジェクトを標準化するかどうか
saltremove: bool = True # MOLオブジェクトを脱塩するかどうか
uncharge: bool = True # MOLオブジェクトを中和するかどうか
# 固定
df: pd.DataFrame = None
names: List[str] = field(default_factory=list)
def data_loading(self):
self.df = pd.DataFrame()
if self.data_file:
with open(self.data_file, 'r') as f:
reader = list(csv.reader(f))[1:] # 1行目がカラムなので
smiles_list = [row[0] for row in reader]
y = [float(row[1]) for row in reader]
self.df[self.task] = y
else:
reader = sys.stdin
smiles_list = [row.split(",")[0] for row in reader if row.split(",")[0] != 'SMILES']
#print(smiles_list)
#return smiles_list
self.df['SMILES'] = smiles_list
def _normalize(self, mol):
normalizer = MolStandardize.normalize.Normalizer()
mol = normalizer.normalize(mol)
return mol
def _saltremove(self, mol):
lfc = MolStandardize.fragment.LargestFragmentChooser()
mol = lfc.choose(mol)
return mol
def _uncharge(self, mol):
uc = MolStandardize.charge.Uncharger()
mol = uc.uncharge(mol)
return mol
def _mol2img(self, mol, mode='std', img_size = 96, res = 0.5, max_len = 300):
embed = int(img_size * res /2)
cmol = Chem.Mol(mol.ToBinary())
cmol.ComputeGasteigerCharges()
AllChem.Compute2DCoords(cmol)
atom_coords = cmol.GetConformer(0).GetPositions()
img = np.zeros((img_size, img_size, 1))
# Compute bond properties
try:
if mode == 'std':
img = np.zeros((img_size, img_size, 1))
bond_props = np.array([[2.0, bond.GetBeginAtomIdx(),
bond.GetEndAtomIdx()] for bond in mol.GetBonds()])
# Compute atom properties
atom_props = np.array([[atom.GetAtomicNum()] for atom in cmol.GetAtoms()])
bond_props = bond_props.astype(np.float32)
atom_props = atom_props.astype(np.float32)
else:
img = np.zeros((img_size, img_size, 4))
# Compute bond properties
bond_props = np.array([[
bond.GetBondTypeAsDouble(),
bond.GetBeginAtomIdx(),
bond.GetEndAtomIdx()
] for bond in mol.GetBonds()])
# Compute atom properties
atom_props = np.array([[
atom.GetAtomicNum(),
atom.GetProp("_GasteigerCharge"),
atom.GetExplicitValence(),
atom.GetHybridization().real,
] for atom in cmol.GetAtoms()])
bond_props = bond_props.astype(np.float32)
atom_props = atom_props.astype(np.float32)
partial_charges = atom_props[:, 1]
#if np.any(np.isnan(partial_charges)):
# return np.array([])
frac = np.linspace(0, 1, int(1 / res * 2))
# Reshape done for proper broadcast
frac = frac.reshape(-1, 1, 1)
bond_begin_idxs = bond_props[:, 1].astype(int)
bond_end_idxs = bond_props[:, 2].astype(int)
# Reshapes, and axes manipulations to facilitate vector processing.
begin_coords = atom_coords[bond_begin_idxs]
begin_coords = np.expand_dims(begin_coords.T, axis=0)
end_coords = atom_coords[bond_end_idxs]
end_coords = np.expand_dims(end_coords.T, axis=0)
# Draw a line between the two atoms.
# The coordinates of this line, are indicated in line_coords
line_coords = frac * begin_coords + (1 - frac) * end_coords
# Turn the line coordinates into image positions
bond_line_idxs = np.ceil(
(line_coords[:, 0] + embed) / res).astype(int)
bond_line_idys = np.ceil(
(line_coords[:, 1] + embed) / res).astype(int)
# Set the bond line coordinates to the bond property used.
img[bond_line_idxs, bond_line_idys, 0] = bond_props[:, 0]
# Turn atomic coordinates into image positions
atom_idxs = np.round(
(atom_coords[:, 0] + embed) / res).astype(int)
atom_idys = np.round(
(atom_coords[:, 1] + embed) / res).astype(int)
# Set the atom positions in image to different atomic properties in channels
img[atom_idxs, atom_idys, :] = atom_props
except :
sys.stderr.write(Chem.MolToSmiles(mol)+'\n')
return img
@property
def RDKitDescriptor(self):
if not 'SMILES' in self.df.columns:
self.data_loading()
self.names = [x[0] for x in Descriptors._descList]
calc = MoleculeDescriptors.MolecularDescriptorCalculator(self.names)
descriptors = [np.array(list(np.nan for i in range(len(self.names)))) if mol is None else calc.CalcDescriptors(mol) for mol in tqdm(self.mol_list)]
IPC = [np.nan if mol is None else Descriptors.Ipc(mol, avg=True) for mol in tqdm(self.mol_list)]
tmp = pd.DataFrame(descriptors, columns=self.names)
tmp['Ipc'] = pd.Series(IPC,name='Ipc')
self.df[self.names] = tmp
return tmp.values
@property
def ChemImage(self):
#https://github.com/deepchem/deepchem/blob/master/deepchem/feat/molecule_featurizers/smiles_to_image.py#L11-L165
if not 'MOL' in self.df.columns:
self.mol_list
max_len = max(len(smiles) for smiles in self.smiles_list)
self.df['ChemImage'] = self.df['MOL'].apply(lambda mol: self._mol2img(mol=mol, max_len=max_len))
return np.array(list(self.df['ChemImage']))
@property
def smiles_list(self) -> pd.DataFrame:
if not 'SMILES' in self.df.columns:
self.data_loading()
return self.df['SMILES'].to_list()
@property
def y(self) -> pd.DataFrame:
if not 'SMILES' in self.df.columns:
self.data_loading()
return np.array(self.df[self.task].to_list())
@property
def mol_list(self):
if not 'SMILES' in self.df.columns:
self.data_loading()
elif not 'MOL' in self.df.columns:
self.df['MOL'] = self.df['SMILES'].apply(Chem.MolFromSmiles)
mol_list = self.df['MOL'].to_list()
if self.preprocessing:
if self.normalize:
mol_list = [None if mol is None else self._normalize(mol) for mol in mol_list]
if self.saltremove:
mol_list = [None if mol is None else self._saltremove(mol) for mol in mol_list]
if self.uncharge:
mol_list = [None if mol is None else self._uncharge(mol) for mol in mol_list]
if not (self.normalize or self.saltremove or self.uncharge):
pass
self.df['MOL'] = mol_list
else:
pass
return self.df['MOL'].to_list()
"""
@property
def feature_list(self, feature, function):
if not 'SMILES' in self.df.columns:
self.data_loading()
elif not feature in self.df.columns:
self.df[feature] = self.df['SMILES'].apply(function)
else:
pass
return self.df['feature'].to_list()
"""
@property
def get_df(self):
if not 'SMILES' in self.df.columns:
self.data_loading()
if 'MOL' not in self.df.columns:
self.mol_list
if not self.names:
self.RDKitDescriptor
if 'ChemImage' not in self.df.columns:
self.ChemImage
return self.df
使い方
サンプルデータのダウンロード
Ames試験のオープンソースデータを保存して一部抜粋しました。
# https://doc.ml.tu-berlin.de/toxbenchmark/
dataset = pd.read_csv('Mutagenicity_N6512.csv')
data = pd.DataFrame()
data['SMILES'] = dataset['Canonical_Smiles'] \
.str.replace('\\','') \
.str.replace('/','') \
.iloc[:100]
data['y'] = dataset['Activity']
data.to_csv('data.csv', index=False)
display(data.head())
| SMILES | y | |
|---|---|---|
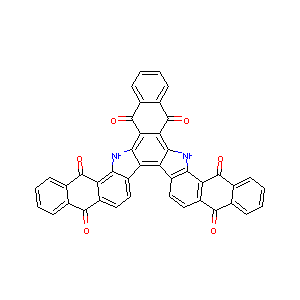
| 0 | O=C1c2ccccc2C(=O)c3c1ccc4c3[nH]c5c6C(=O)c7cccc... | 0 |
| 1 | NNC(=O)CNC(=O)C=N#N | 1 |
| 2 | O=C1NC(=O)C(=N#N)C=N1 | 1 |
| 3 | NC(=O)CNC(=O)C=N#N | 1 |
| 4 | CCCCN(CC(O)C1=CC(=N#N)C(=O)C=C1)N=O | 1 |
データ生成
data_file: 保存したcsvデータのファイル名
task: yに当たるカラム名
SMILES: SMILES。そのまま。
MOL: SMILESから生成したMOLオブジェクト
MaxEStateIndex~fr_urea: RDKit記述子
ChemImage: 原子位置情報
data_file = 'data.csv'
task = 'Activity'
dataset = data_loader(data_file, task=task)
dataset.data_loading()
df = dataset.get_df
display(df.head())
表データ
| Activity | SMILES | MOL | MaxEStateIndex | MinEStateIndex | MaxAbsEStateIndex | ... | MinAbsEStateIndex | qed | MolWt | fr_unbrch_alkane | fr_urea | ChemImage | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | O=C1c2ccccc2C(=O)c3c1ccc4c3[nH]c5c6C(=O)c7ccccc7C(=O)c6c8[nH]c9c%10C(=O)c%11ccccc%11C(=O)c%10ccc9c8c45 | 14.4310727 | -0.3785752 | 14.4310727 | ... | 0.12898832 | 0.18458152 | 646.614 | 0 | 0 | [[[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] ... [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]]] | |
| 1 | 1 | NNC(=O)CNC(=O)C=N#N | 10.4221967 | -0.6702778 | 10.4221967 | ... | 0.24865741 | 0.10384883 | 157.133 | 0 | 0 | [[[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] ... [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]]] | |
| 2 | 1 | O=C1NC(=O)C(=N#N)C=N1 | 10.5217593 | -0.7524074 | 10.5217593 | ... | 0.27814815 | 0.34214759 | 138.086 | 0 | 1 | [[[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] ... [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]]] | |
| 3 | 1 | NC(=O)CNC(=O)C=N#N | 10.3193056 | -0.665463 | 10.3193056 | ... | 0.25907407 | 0.26686523 | 142.118 | 0 | 0 | [[[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] ... [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]]] | |
| 4 | 1 | CCCCN(CC(O)C1=CC(=N#N)C(=O)C=C1)N=O | 11.230461 | -0.9762443 | 11.230461 | ... | 0.034298 | 0.24183825 | 264.285 | 0 | 0 | [[[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] ... [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]] [[0.] [0.] [0.] ... [0.] [0.] [0.]]] |
画像データ
Chem.Draw.MolToImage(df['MOL'][0])
原子位置情報
plt.imshow(df['ChemImage'][0])
解説
data_loaderをインスタンス化し、各種メソッドでSMILESからデータを生成します。
get_dfメソッドで一発で生成できますが、個別にも取ってこられます。
RDKit記述子の生成はPandasToolsを使えばもっと高速化すると思いますが、ちまちま生成する方が中間処理(エラー処理や化合物の前処理など)で色々できるので、個人的には好きです。
原子位置情報を訓練データとしてCNNなどのモデルに学習させることが可能みたいです4。
分子フィンガープリントやMordred記述子など、他のデータも同様に生成できると思います。
deepchemでは様々な記述子の生成が楽に実装できるので、組み合わせると色々できそう。
ただし、エラーが頻発する印象が強い。
以上、お読みいただきありがとうございました。
-
第43回分子生物学会年会フォーラム2F-11「インシリコ創薬を支える最先端情報科学」から抜粋したAlphaFold2の話 ↩
-
SMILES記法は化学構造の線形表記法
SMILESデータを用いることで比較的簡単に回帰や分類問題を解くことができるので、しばしばケモインフォマティクス入門に取り挙げられます3。 ↩ -
Chemception
ただし、数千規模のデータだと無理そうでした。 ↩